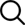Lithium iron phosphate battery is referred to as lithium iron battery. LePO4 with olivine structure is used as the positive pole of the battery, which is connected with the positive pole of the battery by aluminum foil, and the middle is the separator of polymer, which separates the positive pole from the negative pole, but lithium ions can pass through but electrons cannot. The negative pole of the battery is composed of graphite on the right. Between the upper and lower ends of the battery by copper foil and the negative pole of the battery is an electrolyte. The lifepo4 battery 48v is sealed by a metal shell. When the battery is charged, The lithium quotient in the positive electrode migrates to the negative electrode through the polymer membrane; In the discharge process, the lithium ions in the negative electrode migrate to the positive electrode through the diaphragm. The lithium ion battery is named because the lithium quotient moves back and forth during charging and discharging
During charging, under the action of electric field force, the lithium quotient enters the electrolyte from the surface of lithium iron phosphate, passes through the diaphragm, and then migrates to the surface of graphite crystal, and is embedded in the graphite lattice. After lithium quotient is deinked from lithium iron phosphate, lithium iron phosphate is converted into iron phosphate
During discharge, lithium ions are disembedded from the graphite crystal, enter the electrolyte, pass through the diaphragm, migrate to the surface of lithium iron phosphate crystal, and then re embed into the lattice of lithium iron phosphate.