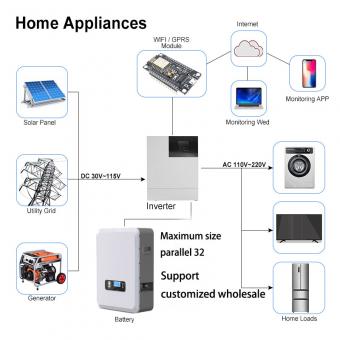
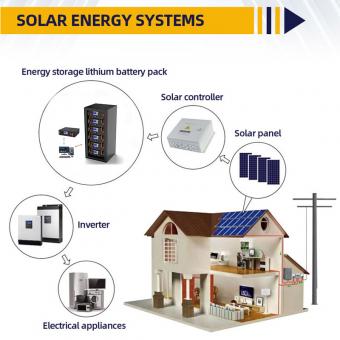
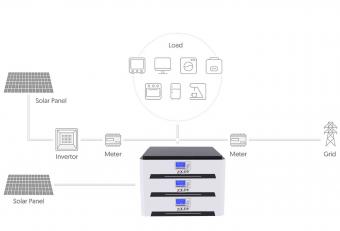
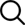Which is better, lithium iron phosphate battery or ternary lithium battery?
If the battery pack is compared to a human body, the module is the "heart", which is responsible for storing and releasing energy and providing power for the car. Compared with the lithium iron phosphate material, the ternary material has higher discharge specific capacity and higher average voltage. Therefore, the mass specific energy of the ternary battery is generally higher than that of lithium iron phosphate battery.
In addition, due to the low true density, small particle size and carbon coating of the lithium iron phosphate material, the compacted density of the pole piece is about 2.3-2.4g/cm3, while the compacted density of the ternary pole piece can reach 3.3-3.5g/cm3. Therefore, the volume specific energy of the ternary material and the battery is also much higher than that of lithium iron phosphate.
From the perspective of safety, the main structure of the lithium iron phosphate material is PO4, and its bond energy is much higher than the M-O bond energy of the ternary material Mo6 octahedron. The thermal decomposition temperature of the fully charged lithium iron phosphate material is about 700 ℃, while the thermal decomposition temperature of the corresponding ternary material is 200-300 ℃, so the lithium iron phosphate material is more safe.
From the perspective of battery, the lithium iron phosphate battery can pass all safety tests, while the needle pricking and overcharging tests of the ternary battery cannot pass easily. It needs to be improved from the structural parts and battery design end.
However, in terms of power performance, the activation energy of Li + in lithium iron phosphate material is only 0.3-0.5ev, resulting in the Li + diffusion coefficient in the order of 10-15-10-12cm2 / s. Extremely low electronic conductivity and lithium ion diffusion coefficient lead to poor power performance of LFP. The Li + diffusion coefficient of the ternary material is about 10-12-10-10cm2 / s, and the electronic conductivity is high. Therefore, the ternary battery has better power performance.
The low electronic conductivity and ionic conductivity of lithium iron phosphate lead to poor low-temperature performance of lithium iron phosphate battery. Compared with normal temperature, the capacity retention rate of lithium iron phosphate battery at - 20 ℃ is only about 60%, while the ternary battery of the same system can reach more than 70%.
Ternary materials contain rare metals such as Ni and Co, and their cost is higher than that of lithium iron phosphate. With the improvement of materials and battery technology, the cost of ternary and lithium iron phosphate batteries has dropped significantly. At present, the market price of ternary batteries is higher than that of lithium iron phosphate batteries. At the same time, the Ni and co elements in the ternary materials and batteries have greater environmental pollution than the environment-friendly Fe and P elements. Combined with the above factors, the demand for environmental control and waste recycling of ternary materials and batteries is more urgent. Therefore, the cost of ternary lithium battery is generally higher than that of lithium iron phosphate battery.